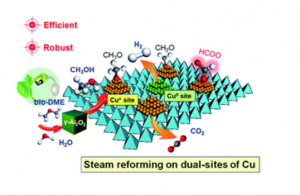
Catalytic reforming provides a practical technique for on-board hydrogen production in fuel cell applications. The high energy density, easy transportation and non-toxicity of biomass-derived dimethyl ether (bio-DME) offer potential to replace methanol for on-board steam reforming (SR). Presently, the reaction mechanism over conventional Cu-based SR catalysts remains elusive, limiting the rational design of highly efficient reforming systems. Herein, we build a catalytic system for bio-DME SR with dual-sites of Cu species, i.e., Cu+ and Cu0 sites, and achieve a record-high H2 production rate of 1145 mol kgcat−1 h−1. Via regulating the ratios of the dual-sites of Cu, we clearly describe molecular understandings on SR. And we discover that the substantially boosted activity is induced by a new Cu+-determined reaction path substituting the conventional Cu0-determined path. Intrinsically, Cu2O can act as a physical spacer and hydroxyl consumer to suppress the aggregation of metallic Cu species in SR. Due to the unique structure of metallic Cu surrounded by Cu2O, the catalyst exhibits robust catalytic performance even after severe thermal treatment. These findings open a new avenue for designing efficient catalytic reforming systems with commercial potential.
Read more in our recent publication:
Achieving efficient and robust catalytic reforming on dual-sites of Cu species
Chem. Sci. 2019, in press. http://doi.org/10.1039/C9SC00015A