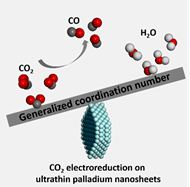
Electrochemical conversion of carbon dioxide (CO2) to value-added products is a promising way to solve CO2 emission problems. Thanks to the eminent conductivity and proper adsorption to intermediates, palladium has become a promising candidate for CO2 electroreduction (CO2ER). However, Pd-based nanocatalysts generally need a large overpotential to overcome energy barriers in generating highly selective products such as CO. This paper describes ultrathin palladium nanosheets effectively reducing the onset potential for CO via exposing abundant atoms with comparatively low generalized coordination number. Hexagonal Pd nanosheets with 5 atomic thickness and 5.1 nm edge length reached CO faradaic efficiency of 94% at -0.5 V, without any decay after a stability test of 8 hours. It appears to be the most efficient among all of Pd-based catalysts toward CO2ER. Uniform hexagonal morphology made it reasonable to build models and take DFT calculations. Both experimental and theoretical explorations indicate that the enhanced activity origins from mainly edge sites on palladium nanosheets.
Read more in our recent publication:
Low-Coordinated Edge Sites on Ultrathin Palladium Nanosheets Boost CO2 Electroreduction Performance
Angew. Chem. Int. Ed. 2018, in press (VIP Paper), DOI: 10.1002/anie.201806432